| Presently running projects | |
|---|---|
| - Metalloregulatory
proteins Many metal ions, including transition metal ions play important roles in biochemical processes. There are others, however, that are toxic for all living organisms. Even the essential metal ions can induce adverse effects when their concentration exceeds an optimal concentration range. It is essential for all living organisms to control the concentration of metal ions and to operate detoxification systems against the toxic constituents. The homeostatic metal ion balance is ensured by the ensemble of various transportation, transformation, storage and regulation processes. Metal ion sensing regulatory proteins, operating at the level of genetic transcription, play a fundamental role in bacterial metal ion homeostasis. These metalloregulatory proteins respond to the change of metal ion concentration by inducing or repressing the expression of proteins that participate in processes that balance the level of the regulated metal ion. Our research focuses mainly on members of the large MerR and ArsR protein families, but most importantly our main goal is to better understand the metal ion selective mechanism of operation of the copper efflux regulator CueR. Our studies involve experiments on the metal ion interaction of model peptides inspired by the metal binding loops these metalloregulators, but also the investigation of the metal ion binding features of the native proteins and their carefully designed mutants. The metal ion regulating/sensing mechanisms of the studied proteins may also be exploited for the design of genetically modified bacteria that (over)produce fluorescent proteins in a metal-ion promoted event and thus may signal the presence or even the change of concentration of a specific metal ion. |
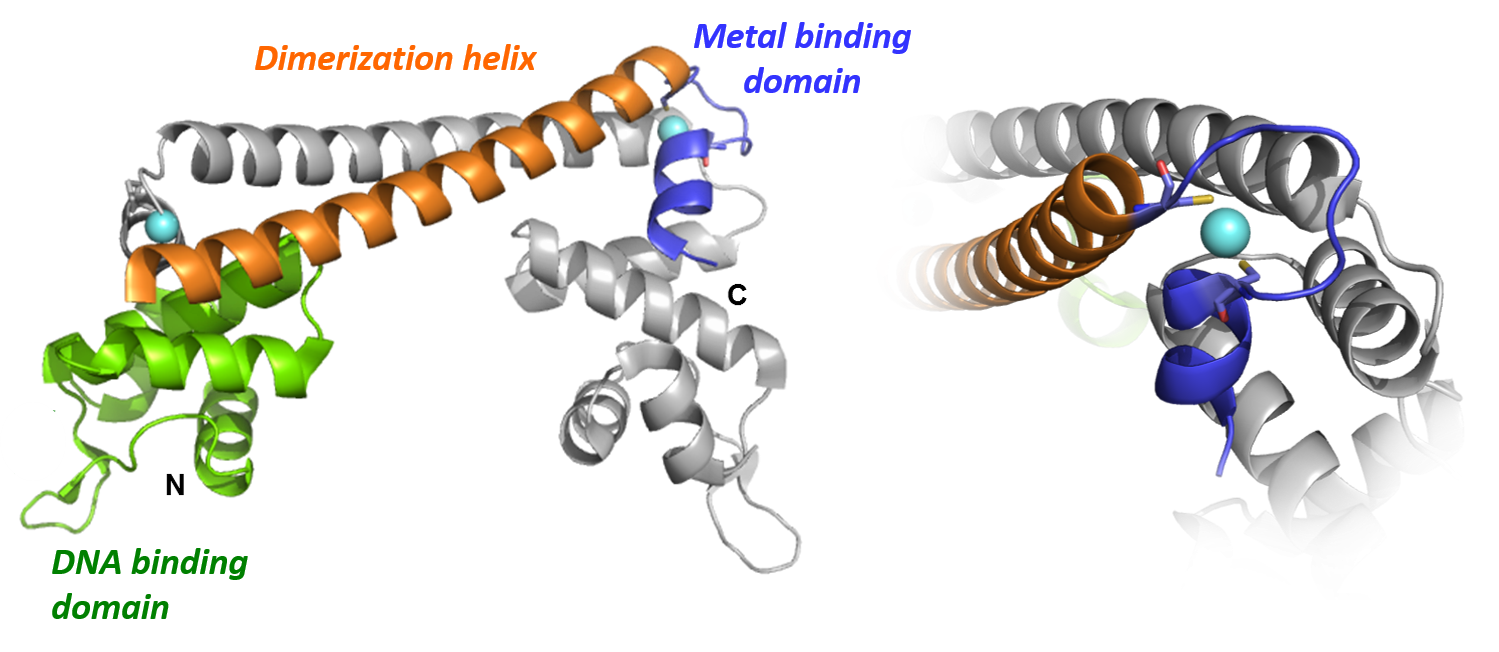 Overall structure highlighting the major domains of the CueR protein (left) and its metal ion binding loop (right) (PDB: 1q05) 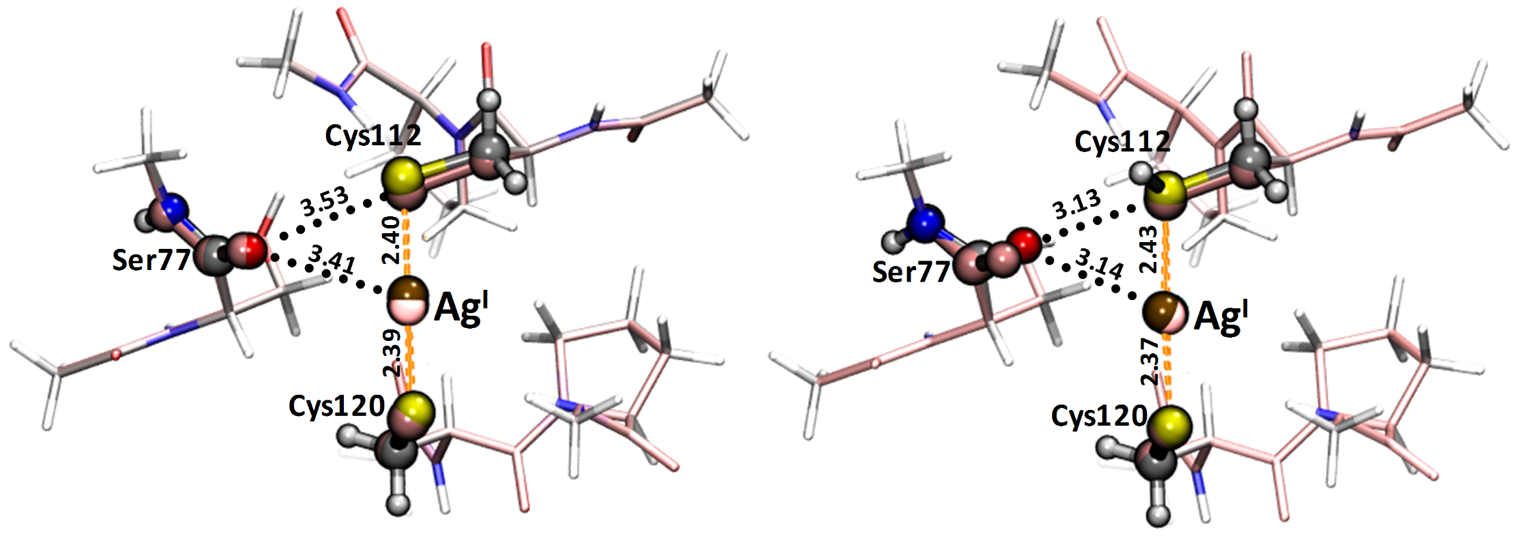 Experimentally
determined structure of CueR-AgI
(pink, PDB 1q06) overlaid with the geometry
optimized structures of the metal ion
binding site involving the coordinated
Cys112 residue in its protonated (right) or
deprotonated form (left)
D.
Szunyogh, H. Szokolai, P.W. Thulstrup, F.H.
Larsen, B. Gyurcsik, N.J. Christensen, M.
Stachura, L. Hemmingsen, A. Jancsó,
"Specificity of the Metalloregulator CueR for
Monovalent Metal Ions: Possible Functional
Role of a Coordinated Thiol?", Angew.
Chem. Int. Ed., 54, 15756-15761
(2015).
|
|
|
|
| -
Peptides
as potential metal ion sensors
Heavy
metal ions appear in the environment from natural or
anthropogenic sources and
present a hazard to all living organisms. The
development of novel methods that
allow the sensitive, fast, simple, sometimes even
on-site determination of such
contaminants, and thus being real alternatives to the
robust, lab-based
instrumental techniques, is in the focus of
environmental/(bio)analytical
research. Our research aims at the design and
investigation of oligopeptides
with efficient toxic (semi)metal ion binding abilities
that may potentially act
as metal ion receptors in optochemical sensors. The
sequences of these ligands
have been inspired by the metal ion binding sites of
various metalloproteins
with transport, chaperone or regulatory functions. The
ligands are synthesized
with fluorophore labels allowing the optical detection
of metal ion binding.
Besides, we are also aiming at the synthesis and
investigation of such
molecular probes in their immobilized, solid-supported
forms (attached to a
resin, glass or quartz surface).
|
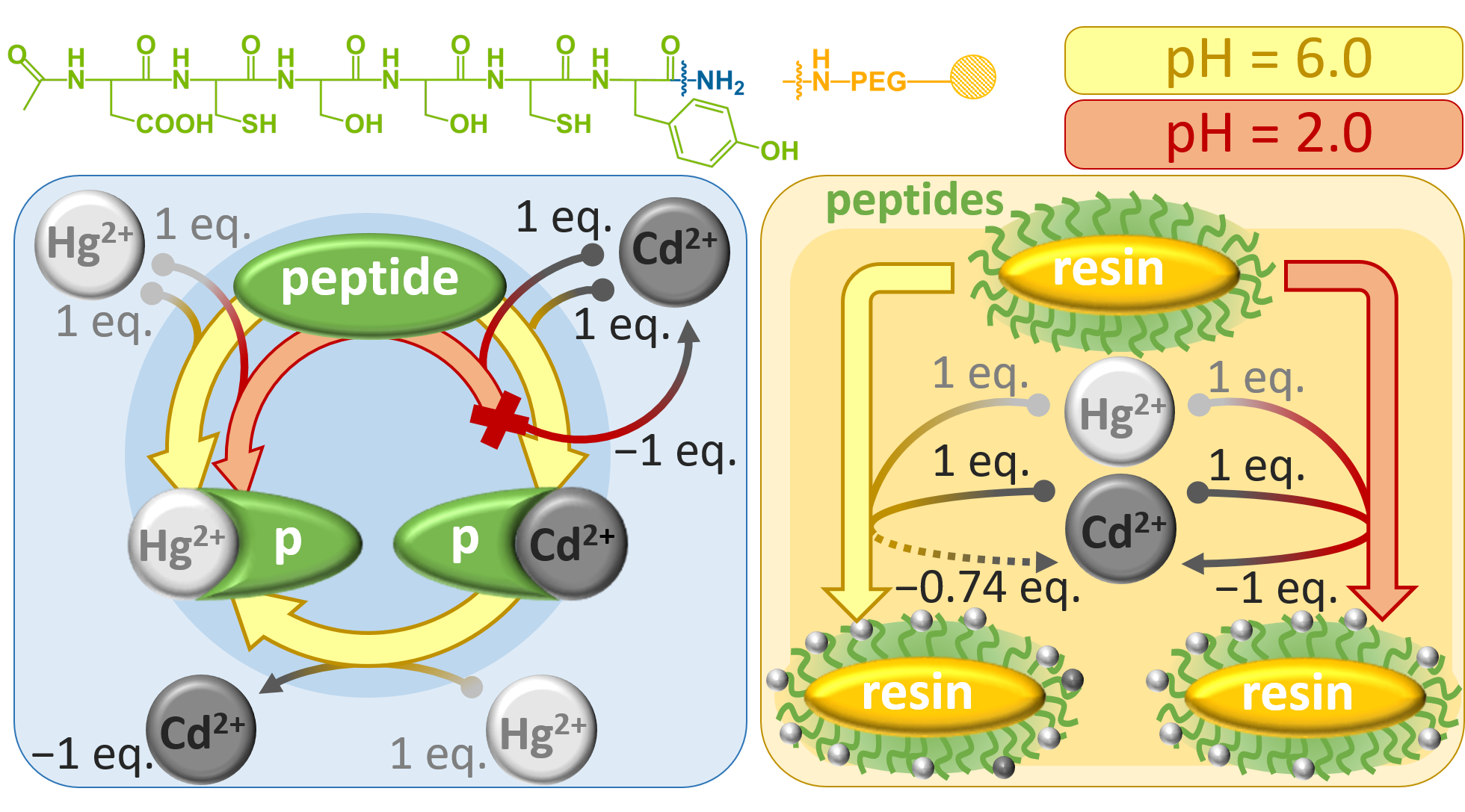 Hg2+
és Cd2+ binding
scheme of a fluorescent hexapeptide, containing
two cysteine units, under acidic (pH = 2.0) and
slightly acidic (pH = 6.0) conditions at
solution phase and in a resin-attached
immobilized form L.I. Szekeres, S. Bálint, G. Galbács, I. Kálomista, T. Kiss, F.H. Larsen, L. Hemmingsen, A. Jancsó, “Hg2+ and Cd2+ binding of a bioinspired hexapeptide with two cysteine units constructed as a minimalistic metal ion sensing fluorescent probe”, Dalton Trans., 48, 8327-8339 (2019). |
|
|
|
| - Interaction
of AsIII (and SbIII)
with thiol ligands: biospeciation,
biochemical effects, chelator
development
Arsenic is
known to exert severe biological
effects on cells and tissues depending on the level
and duration of exposure.
As an interesting contrast arsenic and its derivatives
have been applied, since
the ancient times, as therapeutic agents e.g. in the
treatment of ulcers,
plague or malaria. Most significantly, arsenic
trioxide (As2O3)
has been shown to possess important antitumor
properties against human acute
promyelocytic
leukemia (APL). It was
suggested that similar mechanisms may
operate both in the therapeutic and toxic activities
of arsenic, nevertheless,
the molecular details of these mechanisms are yet to
be explored. It is well
known, that arsenic, in its trivalent oxidation state,
can bind to reduced
thiols, especially when more thiol groups are
available, and this plays a role
in many of its physiological effects. Nevertheless,
solution speciation studies
on the systems of AsIII and small
multithiol ligands are scarce in
the literature, even though understanding the AsIII-thiol
interaction
at the level of small models could be essential in
unravelling the
versatile, sometimes paradoxical biological effects.
With an aim of exploring
the possible binding site and coordination environment
preferences of AsIII
in biological milieu we investigate the interactions
of arsenous acid (H3AsO3
- the typical aqueous form of AsIII) with
several thiol ligands,
including heavy metal ion chelators and peptides.
|
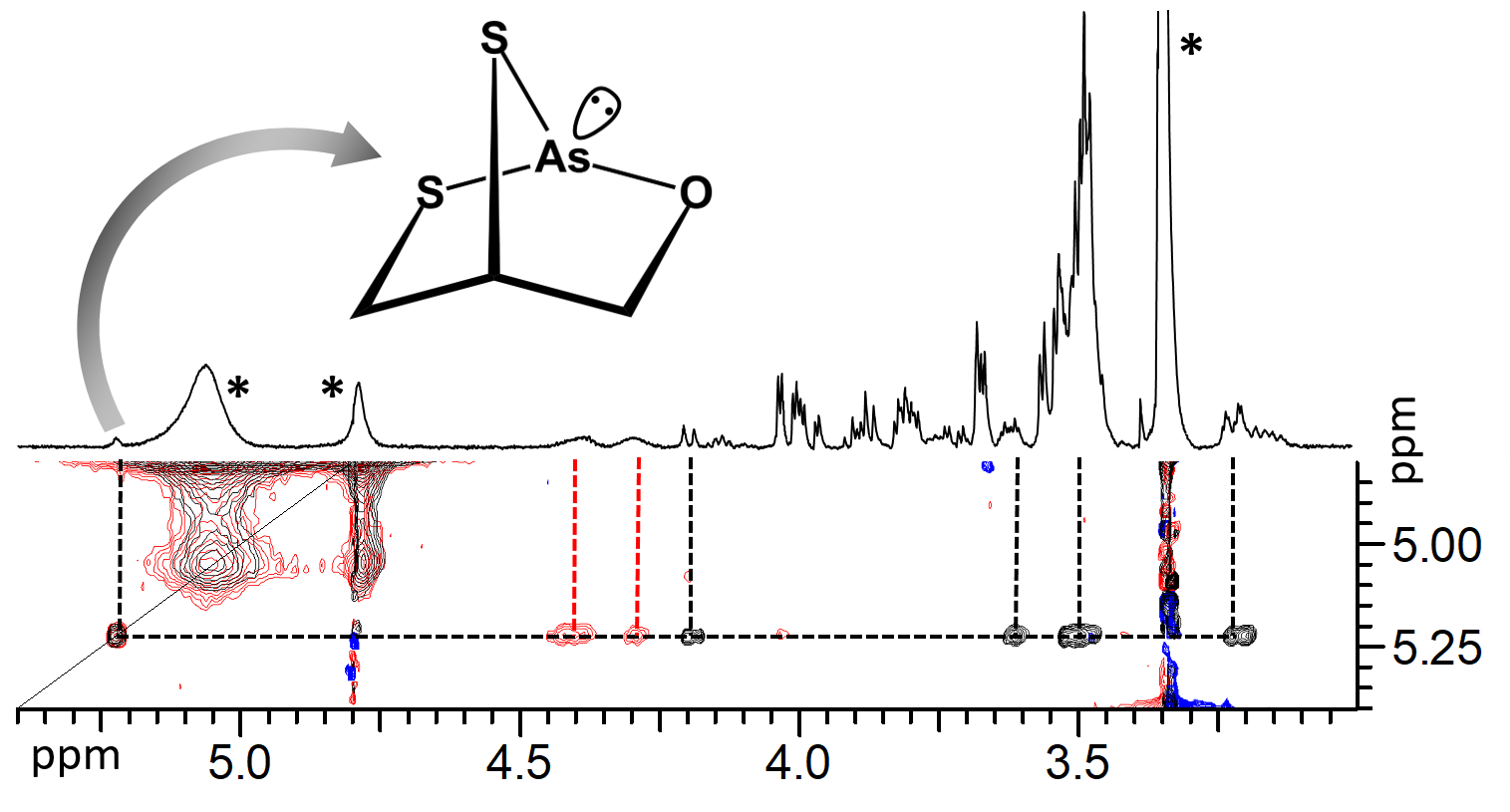 Identification
of a species formed by the binding of two thiol
and an alcoholic hydroxi group in the arsenous
acid - dimercaprol (British anti-Lewisite
- BAL) system by using NMR
spectroscopy 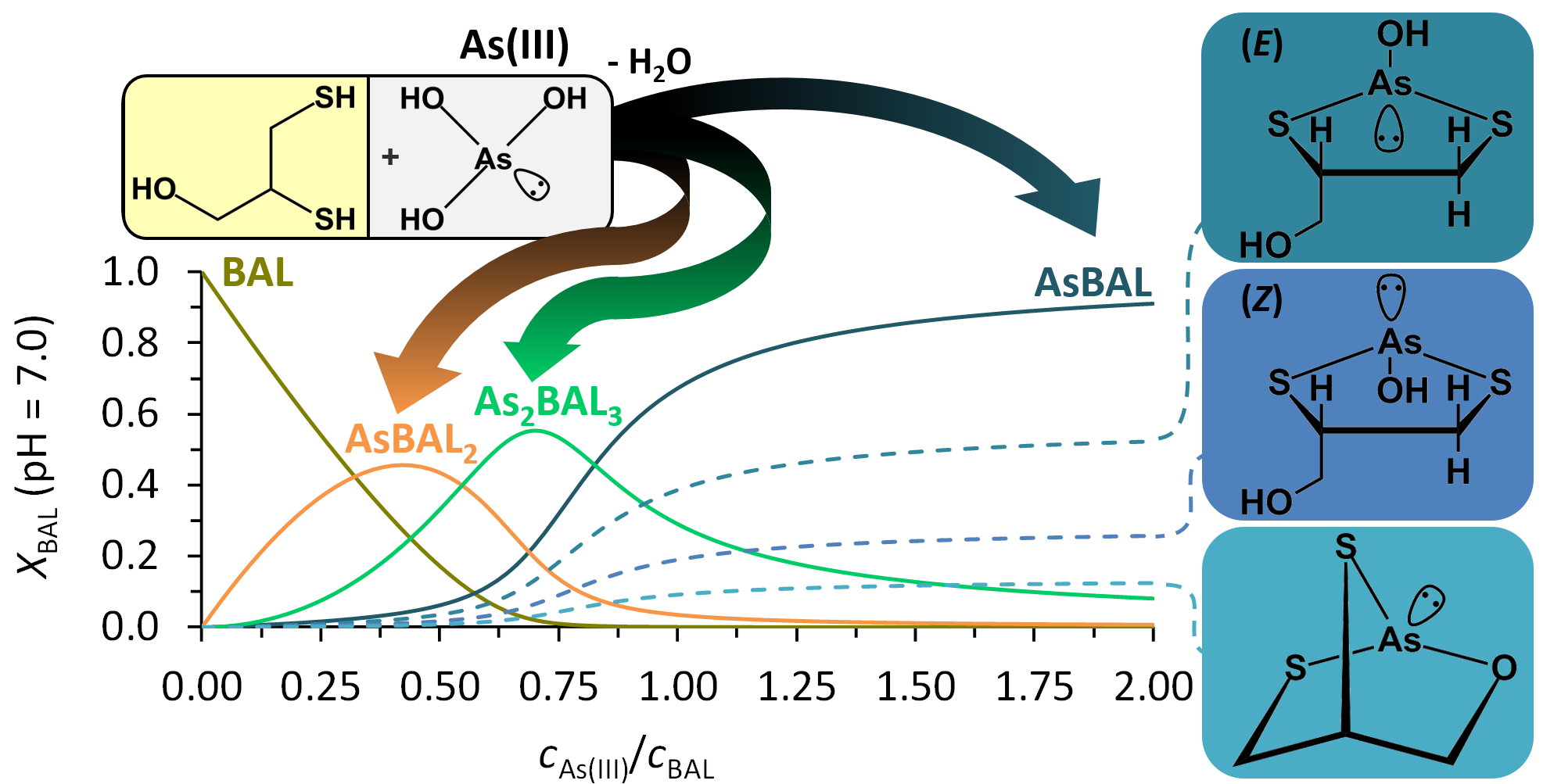 Simulated species distribution curves as a function of the AsIII:BAL concentration ratio an structures of the monomeric microspecies (pH = 7.0, cBAL = 0.5 mM) L.I. Szekeres, B. Gyurcsik, T. Kiss, Z. Kele, A. Jancsó, "Interaction of Arsenous Acid with the Dithiol-Type Chelator British Anti-Lewisite (BAL): Structure and Stability of Species Formed in an Unexpectedly Complex System”, Inorg. Chem., 57, 7191-7200 (2018). |

