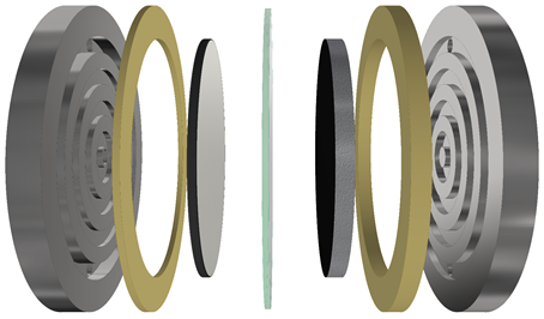
Research topics
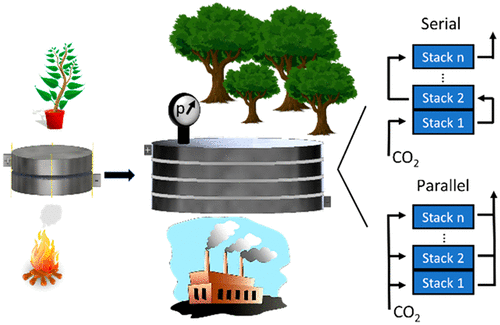
Electrochemical Reduction of CO2
|
Electrochemical reduction of carbon-dioxide is a possible route to turn a harmful waste into valuable raw chemicals, such as carbon monoxide, methane or formic acid. In the past decades the research activity in this field intensified significantly, in parallel with the concern of Society with regards to the increasing carbon dioxide concentration in the atmosphere.
|
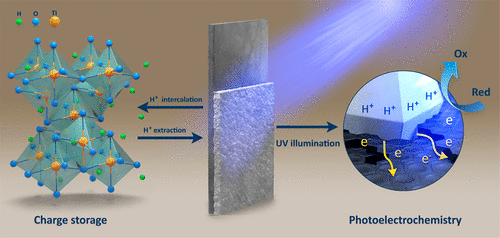
Electrodeposition of Catalyst and Photocatalysts
|
Electrochemical techniques offer a very high level of control over catalyst synthesis on conducting substrates. Tuning either the electrode potential or the current density, the phase composition, particle size, electrode coverage etc. can be easily tuned. Furthermore, the amount of the electrodeposited material is simply controlled by the charge passed, which is directly related to the reaction time. Importantly, the deposition can be stopped, slowed down or varied in many ways during the experiments - this is a truly unique feature of electrochemistry.
|