 | ||
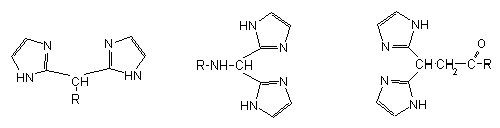
| R = H | R = Ac-Pro-Leu-Gly | R = Ile-Ala-Gly-OEt |
| R = NH2 | R = BOC-Pro-Leu-His | R = His-Ala-Gly-OEt |
| R = CH2-COOH | R = BOC-Pro-His-Gly | R = Ile-His--Gly-OEt |
| R = BOC-His-Leu-Gly | R = Ile-Ala-His-OMe | |
Speciation 98: Abstracts
Katalin Várnagy and Imre Sóvágó
Department of Inorganic and Analytical Chemistry, Lajos Kossuth University, H-4010 Debrecen, Hungary
The ligands containing two or more imidazole rings linked via aliphatic carbon chains can form especially stable complexes with 3d transition metal ions, and they are used to mimick the binding sites of various metalloenzymes, or they can be used as enzyme inhibitors. To study the complex-forming capability of bis(imidazolyl) ligands the simple compounds containing bis(imidazolyl) moiety and different bis(imidazolyl) derivatives of amino acids or tripeptides were prepared.
 | ||
| R = H | R = Ac-Pro-Leu-Gly | R = Ile-Ala-Gly-OEt |
| R = NH2 | R = BOC-Pro-Leu-His | R = His-Ala-Gly-OEt |
| R = CH2-COOH | R = BOC-Pro-His-Gly | R = Ile-His--Gly-OEt |
| R = BOC-His-Leu-Gly | R = Ile-Ala-His-OMe | |
The potentiometric and spectroscopic data obtained for the copper(II) and zinc(II) complexes of the ligands show, that in acidic pH range the bis-aromatic group is the main binding site. It results in the formation of 2N- and 4N-coordinated complexes and the metal ion speciation is influenced only by the weak axial or equatorial coordination of side chain donor groups. However, in the case of amino acid derivatives with terminal amino group the coordination mode changes in higher pH range. The metal ions in the solution are present in complexes with coordination of amino-, amide- and aromatic nitrogen-atoms, and the formation of dimeric species is also possible in some cases.
References
