Speciation 98: Abstracts
Ibolya Török, Tamás Gajda, Tamás Kiss
Department of Inorganic and Analitical Chemistry, József
Attila University, P. O. Box 440, H-6701 Szeged, Hungary
tibolya@chem.u-szeged.hu
The imidazole ring has several unique features which may explain their widespreading utility in nature. For example it often occurs in the active centre of various metalloenzymes. The interaction of transition metal complexes of imidazole containing ligands with relevant biomolecules may provide information for mechanistic details of the enzymatic reactions. Catecholamines play an important role in various neurological and biochemical reactions. The formation of their ternary complexes may be important in the storage and transport of catecholamines in the organism.
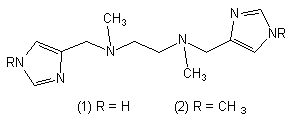
The equilibrium and structural properties of copper(II)- and zinc(II)-complexes of N,N`-dimethyl-N,N`-bis(imidazole-4-ylmethyl)ethane-1,2-diamine (1) and N,N`-dimethyl-N,N`-bis(1-methyl-imidazole-4-ylmethyl)ethane-1,2-diamine (2) in binary and ternary systems with dopamine and L-Dopa have been characterized by potentiometric and spectroscopic (UV-VIS, ESR) methods.

In both copper(II)- and zinc(II)-ligand A systems [A = (1) or (2)] an MA complex is dominant in wide pH range of 2-8 having 2Nim, 2Nterc co-ordination. An MAH-1 complex, probably a mixed hydroxo species, is also formed in both systems above pH~9. In the ternary systems the catechol unit takes also part in metal ion binding disrupting the stable 4N binary complexes. Mixed ligand complexes seem to be formed at pH as low as 2-3 in some systems, and are present in different protonation states (from species MABH4 to MAB) in the whole studied pH range, up to pH~11. EPR studies suggest only bidentate coordination of the imidazole derivatives in the ternary complexes. Their binding modes may be either ethylendiamine-like or histamine-like and isomeric species may be in equilibrium with each other. pH seems to affect the ratio of the two binding isomers.
