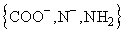 coordination leading
to complexes with a trigonal bipyramidal structure. For Gly-His,
MLH-1 is also formed as the consequence of the deprotonation
of the imidazole ring which is not bound to the dimethyltin cation.
coordination leading
to complexes with a trigonal bipyramidal structure. For Gly-His,
MLH-1 is also formed as the consequence of the deprotonation
of the imidazole ring which is not bound to the dimethyltin cation.
Speciation 98: Abstracts
P. Surdy1,2, P. Rubini1, T. Gajda2 and B. Henry1
1 Laboratoire de Chimie Physique Organique et Colloïdale,
UMR SRSMC CNRS-UHP no 7565, Université Henri
Poincaré-Nancy 1, BP. 239, F-54506, Vandoeuvre-lès-Nancy,
France;
1 Department of Inorganic and Analytical Chemistry, A. József
Universty, 6701 Szeged, P.O. Box 440, Hungary
The exponential increase of the agricultural, industrial and
biological application of the organo-tin (IV) compounds during
the last 50 years has led to their accumulation in the environment
and in biological systems. The binding ability of these species
to biological molecules and to low molecular weight compounds
is important to determine in order to explain their toxicity and
also the potential pharmaceutical application of some dialkyltin
derivatives.
We performed equilibrium (pH-metric) and NMR (1H,
13C and 119Sn) studies of dimethyltin (IV)
complexes of glycyl-glycine (Gly-Gly) and glycyl-histidine (Gly-His)
and of some related ligands.
At low pH (~ 2), MLH (Gly-Gly) and MLH2 (Gly-His) complexes
are formed, the complexing group being the carboxylate group.
Near pH 3-4 a deprotonation occurs due to the formation of hydroxo-mixed
complexes. A further deprotonation is observed between pH 4 and 6
(complex MLH-1 for Gly-Gly and ML for Gly-His). The
NMR results prove a 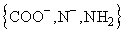 coordination leading
to complexes with a trigonal bipyramidal structure. For Gly-His,
MLH-1 is also formed as the consequence of the deprotonation
of the imidazole ring which is not bound to the dimethyltin cation.
coordination leading
to complexes with a trigonal bipyramidal structure. For Gly-His,
MLH-1 is also formed as the consequence of the deprotonation
of the imidazole ring which is not bound to the dimethyltin cation.
Two important features of these results must be mentioned: (i) the characterization of the metal promoted deprotonation of an amide nitrogen at unexpectedly low pH (4-5) and the role of the carboxylate group which acts as an anchoring group.
