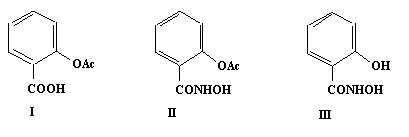 010
010
Speciation 98: Abstracts
Eimear C. O' Brien1, Etelka Farkas2 and Kevin B.Nolan1
1 Department of Chemistry,
Royal Collegeof Surgeons in Ireland, St. Stephen's Green, Dublin
2, Ireland;
2 Department of Inorganic
and AnalyticalChemistry, Lajos Kossuth University, Debrecen, Hungary
Aspirin (I), discovered in 1899, is one of the most widely consumed drugs world-wide. A potent painkiller, aspirin also lowers fever, eases inflammation and evenreduces the risk of heart disease. However long-term aspirin usage canresult in serious side-effects including irritation of the gastric mucosa and renal toxicity. As a result ofthis there is a major interest in the synthesis of aspirin analogues havingthe beneficial therapeutic effects of aspirin without the side-effects.
We decided to attempt the synthesis of the hydroxamic acid analogue ofaspirin (II), a choice prompted by the fact that hydroxamic acids are biologicallyand medically important (e.g. in microbial iron transport, in theinhibition of the nickel-dependent urease and the zinc-dependent matrixmetalloproteinase enzymes). This activity islargely due to the ability of hydroxamic acids to form very stable metalchelates.
 010
010
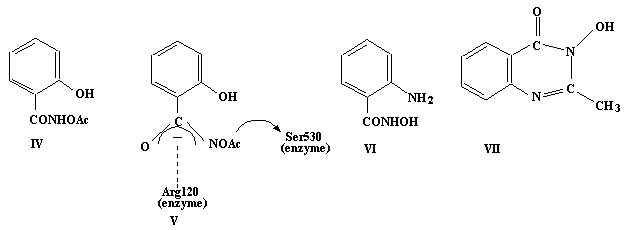
Our attempted synthesis of (II) fromsalicylhydroxamic acid (III) gave O-acetylsalicylhydroxamic acid (IV). Nevertheless the product obtained had a ten-fold greater inhibitoryeffect than aspirin on prostaglandin-H-synthase, a haem enzyme which is thetarget for aspirin activity. Based on the measured pKa value and byanalogy with aspirin we postulate that (IV) inhibits the enzyme by acetyl group transfer asshown in (V). In complexes of (IV), zinc(II) coordinates via the phenolate oxygen and the hydroxamate nitrogen while copper(II) and iron(III) coordination result indeacetylation. Attempted acetylation of anthranilic hydroxamic acid (VI) resulted in the formation of a cyclic hydroxamicacid (VII). Metal complexes of (III) and (VI) were also synthesised and characterised and their metal bindingproperties in solution were studied by potentiometric andspectrophotometric measurements.
Acknowledgement: We thank the Research Committee of theRoyal College of Surgeons in Ireland and Forbairt Ireland (Programme onInternational Collaboration) for financial support.
