Speciation 98: Abstracts
Kevin B. Nolan,1 Eimear O 'Brien1 and Etelka Farkas2
1 Departments of Chemistry, Royal College ofSurgeons in Ireland,
St. Stephen's Green, Dublin 2, Ireland;
2 Lajos Kossuth University, Debrecen, Hungary
Aspirin (O-acetylsalicylic acid) is a widely used analgesic,
antipyretic,anti-inflammatory and anti-thrombotic agent. The
effects of aspirin aredue to inhibition of the haem-containing
enzyme prostaglandin-H-synthase(PGHS). It has however several
side effects sometimes resulting in fatalities and hence there
is a search foralternative drugs with equivalent beneficial therapeutic
effects butlacking the side effects. We therefore attempted
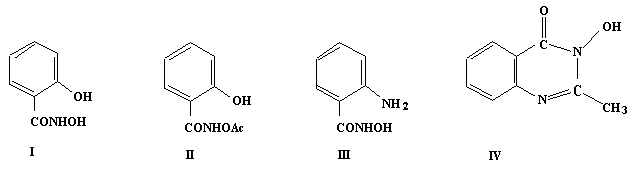
to synthesise ahydroxamic acid analogue of aspirin by acetylating
salicylhydroxamic acid (I). This however resulted inthe
acetylated product (II) in which the hydroxamic acid group
rather than the phenolic group isacetylated. Nevertheless this
compound was found to have a ten-foldgreater inhibitory effect
on PGHS than aspirin. The attempted acetylationof anthranilic
hydroxamic acid (III) resulted in the formation of the
cyclic hydroxamic acid (IV). Since PGHS is a haem enzyme
compound (II) may exert its inhibitoryeffect by binding
to the metal ion. We therefore decided to investigatethe metal
binding properties of (II) and the related hydroxamic acid
ligands, (I), (III) and (IV) as well
as benzohydroxamic acid (V) for comparison. Where solubility
permitted studies were carried out inaqueous solution by pH-metry
and in some cases by visiblespectroscopy.
The species in solution are CuA, CuAH; NiAH, NiAH-1, NiA2H2,
NiA, NiA2H, NiA2; ZnAH,
ZnA2H2, ZnA2,
ZnA, ZnA2H, ZnA2H-1;
FeA, FeAH, FeA2, FeA2H2, FeA3,
where HA represents the hydroxamic acid. Anthranilichydroxamic
acid (III) and benzohydroxamic acid (V) produce
similar species with comparable stability constants indicatingthat
the amino group has little effect on complex formation. The cyclichydroxamic
acid (IV) forms less stable complexes than the acyclicones.

Acknowledgement: We thank the Research Committee of the Royal College of Surgeons in Ireland and Forbairt Ireland (Programme on International Collaboration) for financial support.
