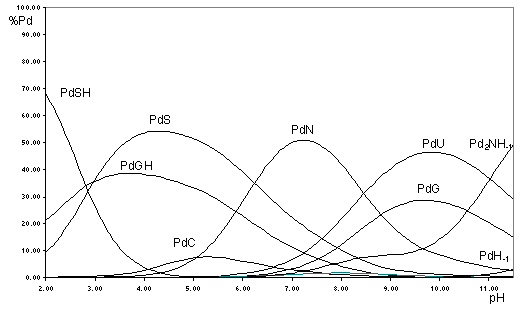
Figure
(Pd: [Pd(dien)]2+, G: 9-Ethylguanine, C: 1-Methylcytosine, U: 1-Methyluracil, N: N-Acetyl-Histidine, S: N-Acetyl-Methionine)
Speciation 98: Abstracts
Zoltán Nagy, István Fábián and Imre Sóvágó
Department of Inorganic and Analytical Chemistry, Lajos Kossuth University, H-4010 Debrecen, Hungary
Ternary complexes of palladium(II) formed in the reaction of [Pd(dien)]2+, [Pd(terpy)]2+, [Pd(GlyAla)] and [Pd(GlyMet)] with N-Ac-Met or GlyMet, were studied by potentiometric, calorimetric, NMR and stopped flow kinetic measurements. The stability constants of the thioether complexes were determined with an indirect potentiometric method, and it was found, that they follow the order:
[Pd(dien)]2+ ~ [Pd(GlyAla)] > [Pd(GlyMet)] > [Pd(terpy)]2+.
The enthalpy values were obtained from calorimetric measurements, and the low thermodynamic stability of thioether complexes of [Pd(terpy)]2+ and [Pd(GlyMet] was explained by steric effects. The comparison of the stability constans of S(thioether)- and N-coordinated complexes support that thioether sulfur donor atoms are the main binding site in acidic media, but they are replaced by N-donors in neutral pH-range. It is demonstrated by the Figure, where metal ion speciation of the system containing [Pd(dien)]2+, N-Acetyl-Methionine, 1-Methylcytosine, 9-Ethylguanine, N-Acetyl-Histidine and 1-Methyluracil in equimolar concentration is given as a function of pH. Fast formation kinetics of the thioether complexes, however, results in the existence of metastable intermediate species.

