 |
Fig. 1
P - HP2 L - HMS-HMS-His |
Speciation 98: Abstracts
P. Mlynarz,1 T. Kowalik-Jankowska,1 M. Stasiak,2 M.T.Leplawy2 and H. Kozlowski1
1 Faculty of Chemistry, University of Wroclaw, 50-383
Wroclaw, Poland;
2 Institute of Organic Chemistry, Technical University,
Lódz, Poland
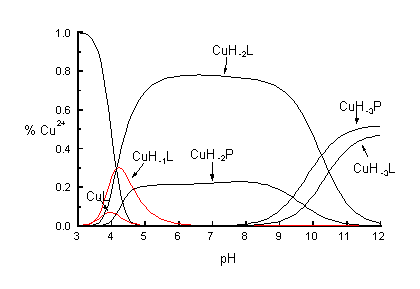
Our recent works on the binding abilities of the peptides containing a-hydroxyl-methylserine (HMS) have shown that this particular amino acid residue stabilises considerably the complex species formed even if its hydroxyl group are not involved directly in the metal ion co-ordination.1 This unusual behaviour of this amino acid residue inspired us to synthesise the new analogue of the albumin N-terminal tripeptide, the major binding site of Cu2+ in human serum albumin. The potentiometric and spectroscopic results clearly shown that HMS-HMS-His is even more potent tripeptide ligand for Cu2+ ions than N-terminal fragments of human protamin HP2 (Arg-Thr-His-Gly-Gln-NH2).2 The N-terminal fragments of HP2 were known up to know as the most efficient oligopeptide binders of Cu2+ ions. The comparison of the stability constants for the Cu2+ complexes clearly show that HMS-HMS-His forms the dominant three orders of magnitudes more stable than Gly-Gly-His and about one order of magnitude more stable than that of HP2 oligopeptide. The species distribution curves calculated for the system of two ligands HMS-HMS-His and Arg-Thr-His-Gly-Gln-NH2 competing with each other in metal ion co-ordination the former ligand binds almost 80% of metal (Fig. 1).
 |
Fig. 1
P - HP2 L - HMS-HMS-His |
References
