Speciation 98: Abstracts
Cu(II) Co-Ordination by a-Hydroxymethylated Deltakephalin
J. Szyrwiel,1 W. Lesniak,1
T. Kowalik-Jankowska,1 M. Stasiak,2 and
M.T. Leplawy
1 Faculty of Chemistry, University of Wroclaw,
50-383 Wroclaw, Poland;
2 Institute of Organic Chemistry, Technical
University, Lódz, Poland
Deltakephalin is a hexapeptide, Tyr-D-Thr-Gly-Phe-Leu-Thr,
very selectively inter-acting with d-opioid receptor. Our earlier
studies have shown that enkephalins and their analogues are very
efficient and highly specific ligands for Cu2+ ions.1,2
Insertion of the a-hydroxymethylserine (HMS) into the peptide sequence
causes the distinct gain in the efficiency in the metal ion co-ordination
and its specificity.3 In this work we have studied
the effect of the a-hydroxymethylserine substitutions into the
deltakephalin sequence on the binding ability towards Cu2+
ions. The effects of Cu2+ on the biological activity
of this type of peptides have also been demonstrated (see refs.
in 3). The potentiometric and spectroscopic data allow
to establish the speciation in deltakephalin and its two analogues
containing HMS residue in the second position, and second and
six positions. The comparison of the stability constants of Cu2+
with three peptides clearly indicate that substitution of HMS
increases distinctly the binding ability of peptide ligand. This
is particularly well seen when HMS was inserted in position two
and six (Table 1, Fig. 1).
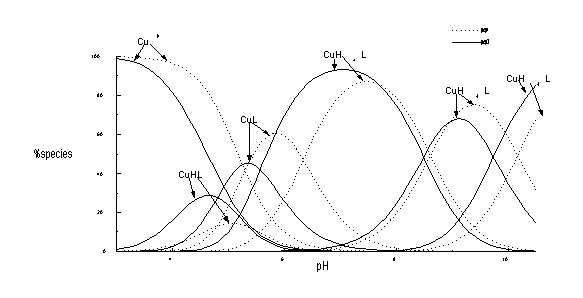
Fig.1
Tabele1
| peptides | logb011
| logb012 | logb013
| logb111 | logb101
| logb1-11 |
logb1-21 | logb1-31
|
| 1) Tyr-D-Thr-Gly-Phe-Leu-Thr
| 9,94 | 17,19
| 20,54 | 14,35
| 9,51 | 3,18
| -5,42 | -15,58
|
| 2) Tyr-HmS-Gly-Phe-Leu-Thr
| 9,76 | 16,90
| 20,37 | 13,90
| 9,66 | 3,77
| -4,61 | -14,71
|
| 3) Tyr-HmS-Gly-Phe-Leu-HmS
| 9,65 | 16,86
| 20,18 | 14,84
| 10,03 | 4,46
| -4,02 | -13,75
|
References
- G.Formicka-Kozlowska, L.D.Pettit, I.Steel, C.E.Livera, G.Kupryszewski,
K.Rolka, J.Inorg.Biochem., 24, 299, (1985)
- T.Kowalik-Jankowska, Ch.O.Onindo, H.Kozlowski, L.D.Pettit,
L.Lankiewicz, M.Jasionowski, J.Inorg.Biochem., 60, 21, (1995)
- T.Kowalik-Jankowska, M.Stasiak, M.T.Leplawy, H.Kozlowski, J.Inorg.Biochem.,
66, 193, (1997)
 Back to the list of abstracts
Back to the list of abstracts
Page created by Attila
Nemes.
Last modified: 21 April 1998
Copyright © Attila Nemes, Debrecen, Hungary. All rights reserved.
URL: http://www.jate.u-szeged.hu/~spec98/abstr/lesniak.html



