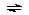 VOA+ + H+
VOA+ + H+
VO2+ + A- 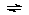 VOA+ or VO(OH)+ + HA
VOA+ or VO(OH)+ + HA 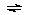 VOA+ + H2O
VOA+ + H2O
Speciation 98: Abstracts
Erzsébet Kiss1, István Fábián1, Tamás Kiss2
1 Department of Inorganic and Analytical Chemistry,
Lajos Kossuth University, H-4010 Debrecen, P.O.B. 21, Hungary;
2 Department of Inorganic and Analytical Chemistry,
Attila József University, H-6701 Szeged, P.O.B. 440, Hungary
Vanadium compounds have been shown to increase glucose transport and oxidation, to stimulate glycogen synthesis in liver and to inhibit gluconeogenesis. Vanadium is also able to mimic most biological effects of insulin in various cell types. Therefore, great efforts have been made to prepare vanadium(IV) and vanadium(V) complexes of high biological activity, low toxicity which are readily absorbed. One promising molecule is bis(maltolato)-oxovanadium(IV) 1. The aim of this work is to follow the kinetics of chemical speciation transformations of this insulin-mimetic drug in various biological fluids and tissues.
In the VO(IV)-maltol system the proton exchange reactions between the bulk water and the different paramagnetic species have been investigated by measuring the spin-spin relaxation time (T2) of water protons. The kinetics of oxovanadium(IV) complex formation reactions with maltol has been studied by the stopped-flow method at 25 oC, I=0.2 M.
Maltol forms mono and bis complexes with the oxovanadium(IV) ion. The first order rate constants of the proton exchange between the bulk water and the paramagnetic maltolato complexes were determined. The significant contribution of the VOA2 complex to the paramagnetic 1H NMR line-broadening confirms that the bis complex is an equilibrium mixture of the cis and trans isomers.
According to our data the following two pathways are operative
in the formation of the VOA+ complex:
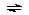 VOA+ + H+
VOA+ + H+
VO2+ + A- 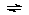 VOA+ or VO(OH)+ + HA
VOA+ or VO(OH)+ + HA 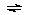 VOA+ + H2O
VOA+ + H2O
It was shown that the first coordinated ligand significantly labilizes the coordinated water molecules. The formation of the VOA2 complex is about one order of magnitude faster than that of the mono complex.
Reference
