 | 
|
Speciation 98: Abstracts
A.Karaczyn1, A.Dobosz2, H.Kozlowski1, T.Yu.Sliva3, I.O.Fritsky3, and J.Swiatek-Kozlowska3
1 Faculty of Chemistry, University of Wroclaw,
50383 Wroclaw, Poland;
2 Medical University of Wroclaw, 51603
Wroclaw, Poland;
3 Department of Chemistry, Shevchenko University,
Kiev 252033, Ukraine
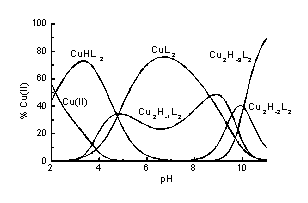
Our recent studies have shown that oximes of amino acids and peptides are very specific and efficient ligands for Cu2+ and Ni2+ ions. The complex species formed are stable, water-soluble and extensive oligomerisation is usually observed above pH 5. The oligomer formation results from the presence of two alternative donor centres at the oxime group (N and O), which both have high affinity for metal ions. The cyanoximes containing strongly electron-withdrawing substituent at a-carbon differ distinctively both in the acidity of oxime group and the complex stability. Deprotonation of the oxime unit allows the {N.O} donor set to form dimeric species which was suggested by study of complex formation with Cu2+ ions.
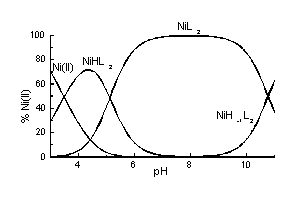
In this poster the solution studies on Cu2+ and Ni2+ complexes with 2-cyano-2-(hydroxyimino)acetic acid (H2L) as well as the first X-ray evidence for the dimeric complex formation are presented. H2L exhibits two protonation constants corresponding to oxime OH (pK = 6.61) and carboxylate (pK = 1.25) functions. Potentiometric and spectroscopic data obtained for Cu2+-containing solutions suggest formation of several species including monomeric CuHL2 and CuL2 as well as dimeric Cu2H-1L2, Cu2H-2L2 and Cu2H-3L2 (Fig.1), the formation of dimeric complexes is supported by the EPR spectra. From the aqueous solution, the dimer Cu2L2 . 6H2O was crystallised, its X-ray analysis revealed the presence of oxime {N,O}2 bridge between the Cu(II) atoms. Ni2+ ions form in solution monomeric complex species only: NiHL2, NiL2 and NiH-1L2 (Fig.2).
 | 
|
