Speciation 98: Abstracts
Attila Jancsó1,*, Etienne Mulliez2 and Tamás Gajda1,*
1 Department of Inorganic and Analytical Chemistry,
József Attila University, P.O.Box 440, H-6701 Szeged, Hungary;
2 Université Joseph Fourier, Laboratorie d'Etudes
Dynamiques et Structurales de la Sélectivite, UMR 5616,
301, rue de la Chimie, 38041-Grenoble cedex, France
email: jancso@chem.u-szeged.hu;
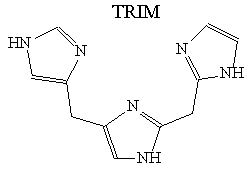
The imidazole moiety is an essential binding site of various metalloproteins, e.g. almost all copper(II) or zinc(II) enzymes contain one or more metal bonded imidazole ring in their active sites. Model compounds mimicking the main feature of active sites are used for a long time to characterise better the relation between structure and function of these enzymes. Following our previous work on co-ordination chemical study of imidazole containing multidentate ligands, we have prepared five new imidazole based ligand. The ligand TRIM (Sceme 1), containing three attached imidazole rings, is a very promising model compound for many copper(II) and zinc(II) containing metalloproteins. In the other four ligands, the imidazole rings of TRIM were replaced by pyridine units and/or amino groups. Potentiometry, UV/VIS, EPR and NMR spect-roscopic methods were used for determining the stability constants and the structures of the species formed in solution. The above structural variety of the ligands allowed us to achieve omparative data between basicity, -donor properties of the nitrogen donors, the size of chelate rings and metal binding properties. In a number of metalloproteins, other donor groups (belonging to the protein or substrate) are also co-ordinated to the metal ion beside the imidazole nitrogens. In this respect, some terner systems of TRIM (the second ligands: L-cysteine and uridine-5'-phosphate) were also investigated for modelling these interactions.

* Acknoledgement: The authors are grateful for the financial support of the Hungarian Research Foundation (OTKA T025114)
