Speciation 98: Abstracts
G. N. L. Jameson, and W. Linert
Institute of Inorganic Chemistry, Technical University of Vienna, Getreidemarkt 9, A-1060 Vienna, Austria
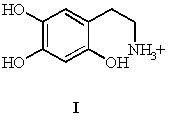
6-hydroxydopamine (2,4,5-Trihydroxyphenylethylamine), I, has been found to have interesting chemical properties which make it biologically important.

It is seen to be oxidised rapidly by iron(III) and this reaction has been studied using stopped-flow kinetics, and low temperature UV/Vis/IR spectroscopy. The kinetic data obtained at low pH provide evidence that the reaction results in a mixture of the ortho- and para- quinones, the para-quinone being in large excess and produced at a rate that precludes prior chelate formation. This 'direct electron transfer' in the case of 6-hydroxydopamine is not remarkable when compared with its structural relative noradrenaline which is seen to react by parallel pathways. The kinetics further show that it reacts with Fe3+ and, much more rapidly, with FeOH2+. This is of particular importance because in the body iron is stored in the protein ferritin as a ferric oxy-hydroxide. Its ability to remove iron from ferritin has previously been shown [1] and may explain its neurological toxicity.
Proton nmr has shown that oxidation of 6-hydroxydopamine is accompanied by the loss of one of the two aryl protons. This reaction appears to be an inter-molecular condensation to form a dimeric species.
Acknowledgements:
This work has been supported by the 'Fonds zur wissenschaftlichen
Forschung in Österreich' (Project no. 11218).
Reference:
