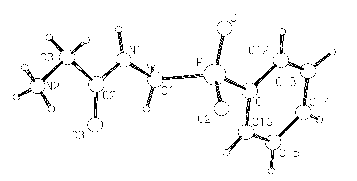
Figure 1: Peptide part of the structure of Gly-AMPPh.H2O
Speciation 98: Abstracts
Petr Hermann, Michal Lukás, Pavel Vojtísek, Jan Rohovec and Ivan Lukes
Department of Inorganic Chemistry, Universita Karlova (Charles University), Albertov 2030, 128 40 Prague 2, Czech Republic
New phosphinic dipeptides of the general formula H2NCH2CONHCH2P(R)(O)(OH) where R = H, Me, Ph and t-Bu has been synthesised by active ester method (Z-Gly-Succ) from the free aminophosphinic acids based on literature. The crystal structure determination of the dipeptide derived from aminomethyl(phenyl)phosphinic acid confirmed a zwitterionic structure expected in the solid state. Bond distances and angels in peptide moiety are virtually the same as in common dipeptides and therefore, are not influenced by the phosphinic group (Figure 1).

Figure 1: Peptide part of the structure of Gly-AMPPh.H2O
Protonation constants were determinated potentiometrically and protonation scheme was confirmed by 1H and 31P NMR titrations. Basicity of amino group is almost the same like in the carboxylic and phosphonic analogues1. On the other hand, acidity of phosphinic moiety is lowered in comparison to the free aminophosphinic acid2. Complexing ability towards metals of the biological interest (Cu(II), Co(II), Ni(II) and Zn(II)) were investigated. The results found for phosphinic dipeptides are discussed and compared with the analogous common dipeptides and dipeptides with terminal phosphonic group.
References
