Speciation 98: Abstracts
T. Gajda1, R. Krämer2, A. Jancsó1
1 Department of Inorganic and
Analytical Chemistry, A. Jozsef University, 6701 Szeged P.O. Box
440, Hungary;
2 Anorganisch-Chemisches
Institute der Universität, Wilhelm-Klemm str. 8, 48149 Münster,
Germany
A number of metalloenzymes that hydrolyse phosphate esters are activated by two or more metal ions. Nucleases are one of the most important class of these enzymes, which are used in the reparation and hydrolysis of DNA and RNA. The hydrolytic cleavage of RNA is a two-step process: the first step is a transesterification forming a cyclic phosphate ester, while the second step is the hydrolysis.
Recently, great efforts have been made to develop efficient artificial nucleases. Most of these studies were made to attain greater hydrolysis rate using metal-ions (e.g. lanthanoids, Co(III)), ligands (polyaza-macrocycles) and test compounds (activated phosphate esters) which do not reflect the active centre of nucleases and native substrates. Only a few metal complexes have been published having structural similarities with the native enzymes.
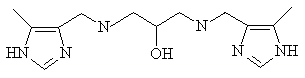
In zinc(II) containing phophatases, metal-bound imidazole rings
are always present. To mimic both structural and functional properties
of above metalloenzymes, we have prepared a binucleating imidazole
ligand (LH4) and studied its zinc(II) complexes.

The equilibrium study of the equimolar Zn(II)-L system revealed the unique formation of the 4N co-ordinated ZnL species in the neutral pH range, which undergoes further deprotonation at pH > 10. In presence of metal excess ( [Zn]/[L] = 2/1 ), however, two strongly overlapped "extra" deprotonation were observed around pH 7.3, forming the Zn2LH-2 (1) complex. The two deprotonation in letter species was assigned to the formation of a m-hydroxo-m-alkoxo bridged metal centres. Parallel with the formation of 1, an important increase of catalytic activity was observed (having a sigmoidal pH-profile) modelling both above mentioned steps of RNA hydrolysis. 1 provides ca. 1400 fold rate acceleration (T = 298 K, pH = 8, [1] = 1 mM) for the intramolecular transesterification of 2-hydroxypropyl-p-nitrophenylphosphate (hpnp), in respect to the metal free system. To mimic the second step of RNA hydrolysis, the non-activated uridine 2',3'-cyclic monophosphate (2',3-cUMP), having real biological relevance, was used as model compound. Interestingly, 1 has been found even more reactive towards the hydrolysis of 2',3'-cUMP as observed with hpnp. Under nearly physiological conditions (T = 310 K, pH = 8, [1] = 2 mM) a ca. 104 fold rate acceleration was detected. A comparison with the literature data shows, that 1 is one of the most reactive zinc(II) containing complex reported to date for promoting both the transesterification and the hydrolysis of 2',3'-cUMP. The possible mechanisms of above reactions are also discussed.
