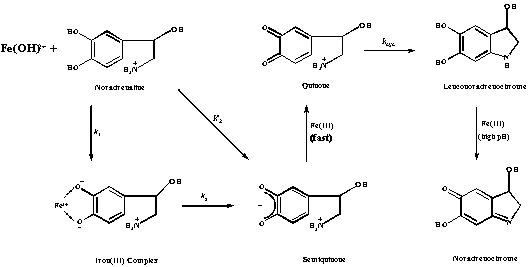
Scheme 1: Overall Route of Oxidation of Noradrenaline
Speciation 98: Abstracts
Usama El-Ayaan1, Reginald F. Jameson2 and Wolfgang Linert*1
1Institute of Inorganic Chemistry, Technical University
of Vienna, Getreidemarkt-9/153, A-1060 Vienna, Austria;
2Department of Chemistry, The University, Dundee DD1 4HN,
Scotland, UK
In anaerobic acid solution noradrenaline [norepinephrine], L-1-(3,4-dihydroxyphenyl)2-(amino)ethanol reacts with Fe(OH)2+ by two parallel reaction pathways to yield iron(II) and the semiquinone: (i) by direct electron exchange (an "outer sphere" reaction). (ii) by initial formation of an iron(III) complex which undergoes internal electron exchange. Subsequent steps yield a quinone, leuoconoradrenochrome and finally the noradrenochrome.

Scheme 1: Overall Route of Oxidation of Noradrenaline
Acknowledgements: Thanks for financial support are due to the Austrian National Bank(Project11218-CHE) and to the "Österreische Akademische Austauschdienst".
