 |
|
Speciation 98: Abstracts
A.Dobosz1, I.Fritsky2, A.Karaczyn3, H.Kozlowski3, N.Dudarenko2, and J.Swiatek-Kozlowska1
1Medical University of Wroclaw, 51603
Wroclaw, Poland;
2Department of Chemistry, Shevchenko University, Kiev 252033, Ukraine;
3Faculty of Chemistry, University of Wroclaw,
50383 Wroclaw, Poland
The most effective modifications of carboxylic group in amino acid donor set leading to very effective family of ligands is the transformation of carboxylate into hydroxamic function. From the other hand, modification of the amino group into the oxime function (HON=) results in a very acidic nitrogen and rather basic oxygen (log K ~ 11.0). This oxime nitrogen binds metal ions at very low pH acting as even more efficient anchoring site than that of amino group. Thus, both hydroxamic and oximic analogues of amino acids are much more efficient in metal ion binding than parent amino acid. In this work we have synthesised and studied the binding ability of the new ligand: 2-(hydroxyimino)-propanohydroxamic acid (HPH) {CH3-C(=NOH)-C(O)-NHOH} containing both oximic and hydroxamic functions as two potential co-ordination sites in one ligand molecule.
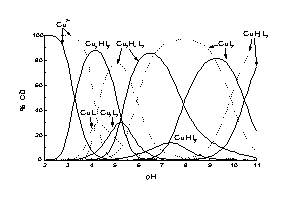
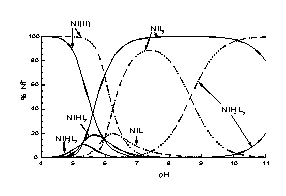
HPH indicates two ionisation constants corresponding to protonation of the hydroxyimino group (pK 11.00) and the hydroxamic group (pK 8.16). Potentiometric data calculations show that HPH forms very stable dimeric species with Cu2+ already below pH 3 which confirms by vanishing of the EPR spectrum in solution around pH 4. Above pH 6 the EPR spectrum reappears with clear hyperfine splitting suggesting the 2x{Nox,Nhydrox-} co-ordination in all monomeric species formed above this pH: CuHL2, CuL2 and CuH-1L2 (Fig. 1). HPH forms also very stable complexes with Ni2+ ions (Fig.2): NiH2L2, NiHL2, NiL2 and NiH-1L2. The stability constants both for Cu2+ and Ni2+ complexes are distinctly higher than those of L--alaninehydroxamic acid and 2-(hydroxyimino)propanoic acid.
 |

|
