Speciation 98: Abstracts
Péter Buglyó1,*, Chris Orvig1, Thomas R. Ward2 and Andreas Lutz2
1Department of Chemistry,
University of British Columbia, 2036 Main Mall, Vancouver,
B.C. V6T 1Z1 Canada;
2Chemistry and Biochemistry
Department, University of Berne, Freiestrasse 3, CH-3012 Berne,
Switzerland;
*On leave from Department of Inorganic and Analytical
Chemistry, Kossuth University, H-4010 Debrecen, Hungary
Both iron deficiency and iron excess are detrimental to living organisms therefore the understanding of the iron uptake and storage mechanisms is crucial.

Due to the extended hydrolysis of iron(III) at physiological conditions, natural iron binders like siderophores must have a very high affinity for Fe(III). One of these, the tris-catecholate enterobactin binds the metal ion with an overall stability constant of logK ~ 49. With such a high complex stability the mechanism of iron-release still remains to be solved.
One of the hypotheses for iron-release is the successive protonation of each catecholate oxygen in the [Fe(III)-enterobactin)]3-, yielding the weaker salicylamide type binding mode. Another possibility may be the in vivo reduction of the metal ion by NADH and the enhanced release of the resulting Fe(II).
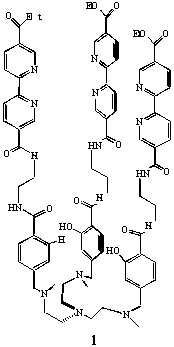
To probe the iron-release mechanism, a dodecadentate tripodal ligand (1) has been synthesized1,2 incorporating a hard tris-salicylamide unit for iron(III) and a softer tris bipyridine cavity capable of binding Fe(II). By modifying its oxidation state, the iron should move from one binding site to the other, yielding a potentially redox-triggered molecular switch3.
We report here the results on the iron(II) and iron(III) binding
ability of ligand 1 and some model compounds using combined
pH-potentiometry and visible spectrophotometry.
References
