Speciation 98: Abstracts
Comparison of the Metal Ion-Binding Properties
of the Nucleotide Analogue, 1-[2-(Phosphonomethoxy)ethyl]cytosine
(PMEC), with its Parent Nucleotide Cytidine 5'-Monophosphate (CMP2-).
Evaluation of Intramolecular Equilibria
Claudia A. Blindauer1
and Antonín Holy2
1Institute of Inorganic Chemistry, University of Basel, Spitalstrasse 51, CH-4056 Basel, Switzerland;
2 Institute of Organic Chemistry
and Biochemistry, Academy of Sciences, CZ-16610 Prague, Czech Republic
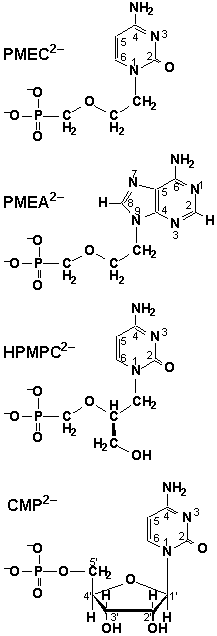 PMEC is structurally related to the highly active antivirals PMEA
(adefovir) and HPMPC (cidofovir), which are particularly effective
against HI-viruses and cytomegaloviruses, respectively [1]. Despite
its close resemblance to these two compounds, PMEC is devoid of
any significant activity against the viruses tested [2]. Enzymic
reactions involving nucleotides, e.g. transphosphorylations or
nucleic acid polymeri-sations, generally require the presence
of divalent metal ions. Consequently, metal ion complexes of nucleotides
like CMP2- were studied intensively
[3]. These studies have recently been ex-tended to nucleotide
analogues in order to compare their properties with those of the
corresponding parent compounds [4,5].
PMEC is structurally related to the highly active antivirals PMEA
(adefovir) and HPMPC (cidofovir), which are particularly effective
against HI-viruses and cytomegaloviruses, respectively [1]. Despite
its close resemblance to these two compounds, PMEC is devoid of
any significant activity against the viruses tested [2]. Enzymic
reactions involving nucleotides, e.g. transphosphorylations or
nucleic acid polymeri-sations, generally require the presence
of divalent metal ions. Consequently, metal ion complexes of nucleotides
like CMP2- were studied intensively
[3]. These studies have recently been ex-tended to nucleotide
analogues in order to compare their properties with those of the
corresponding parent compounds [4,5].
Our results obtained via potentiometric pH titrations provide
insight into the solution structures adopted by such complexes.
For example, the M(PMEC) complexes, where M2+
= Mg2+, Ca2+, Mn2+,
Co2+, Zn2+, Cd2+,
etc., consist of two isomers: i.e., one with the metal ion coordinated
only to the phosphonate group, and a second one forming a five-membered
chelate with the ether oxygen atom (see Structure). No metal ion
interaction involving the cytosine ring could be detected. In
this latter respect, PMEC2- behaves
like its parent nucleotide CMP2-, in
the complexes of which also no interaction with the nucleobase
occurs [3]. The formation degree of the five-membered chelates
in the M(PMEC) species varies between approximately 20 and 70
%.
The engaged and stimulating advice of Professor Helmut Sigel
(H.S.) during the course of this work is gratefully acknowledged.
This study was supported by the Swiss National Science Foundation
(H.S.), the Swiss Federal Office for Education and Science (COST
D8; H.S.), and the Ministry of Education of the Czech Republic
(COST D8; A.H.).
References
- A. Holy, E. De Clercq, I. Votruba, ACS Symp. Ser. 401,
51-71 (1989).
- L. Naesens, R. Snoeck, G. Andrei, J. Balzarini, J. Neyts,
E. De Clercq, Antiviral Chem. Chemother. 8, 1-23
(1997).
- S. S. Massoud, H. Sigel, Inorg. Chem. 27, 1447-1453
(1988).
- C. A. Blindauer, A. H. Emwas, A. Holy´, H. Dvor;áková,
E. Sletten, H. Sigel, Chem. Eur. J. 3, 1526-1536
(1997).
- H. Sigel, Coord. Chem. Rev. 144, 287-319 (1995);
H. Sigel, J. Indian Chem. Soc. 74, 261-271 (1997)
(P. Ray Award Lecture).
 Back to the list of abstracts
Back to the list of abstracts
Page created by Attila
Nemes.
Last modified: 20 April 1998
Copyright © Attila Nemes, Debrecen, Hungary. All rights reserved.
URL: http://www.jate.u-szeged.hu/~spec98/abstr/blindaue.html

 PMEC is structurally related to the highly active antivirals PMEA
(adefovir) and HPMPC (cidofovir), which are particularly effective
against HI-viruses and cytomegaloviruses, respectively [1]. Despite
its close resemblance to these two compounds, PMEC is devoid of
any significant activity against the viruses tested [2]. Enzymic
reactions involving nucleotides, e.g. transphosphorylations or
nucleic acid polymeri-sations, generally require the presence
of divalent metal ions. Consequently, metal ion complexes of nucleotides
like CMP2- were studied intensively
[3]. These studies have recently been ex-tended to nucleotide
analogues in order to compare their properties with those of the
corresponding parent compounds [4,5].
PMEC is structurally related to the highly active antivirals PMEA
(adefovir) and HPMPC (cidofovir), which are particularly effective
against HI-viruses and cytomegaloviruses, respectively [1]. Despite
its close resemblance to these two compounds, PMEC is devoid of
any significant activity against the viruses tested [2]. Enzymic
reactions involving nucleotides, e.g. transphosphorylations or
nucleic acid polymeri-sations, generally require the presence
of divalent metal ions. Consequently, metal ion complexes of nucleotides
like CMP2- were studied intensively
[3]. These studies have recently been ex-tended to nucleotide
analogues in order to compare their properties with those of the
corresponding parent compounds [4,5].

