 | |
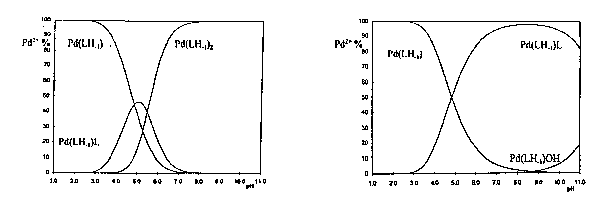
| 1.a. AlaGly/Pd(II) = 2 | 1.b. GlyAla/Pd(II) = 2 |
Speciation 98: Abstracts
Csaba G. Ágoston and Imre Sóvágó
Department of Inorganic and Analytical Chemistry, Lajos Kossuth University, H-4010 Debrecen, Hungary
It has already been well-characterized that palladium(II) effectively binds the ligands containing deprotonated amide nitrogen donor atoms. As a consequence, palladium(II) complexes of peptides are formed in very acidic solutions and stability constants of the corresponding species are scarcely available.
Solution equilibria and structural characterisation of palladium(II)-peptide complexes (GlyGly, a-AlaGly, Gly-a-Ala, b-AlaGly, Gly-b-Ala, PheGly, GlyPhe, Trigly and Tetragly) were carried out in this study with the combined application of potentiometric and 1H-NMR measurements. Potentiometric determination of stability constants required the presence of chloride ion as a competitive ligand.
The formation of the species [PdLCl], [Pd(LH-1)Cl]-, [Pd(LH-1)L]2-, [Pd(LH-1)(OH)]- and [Pd(LH-1)2]2- were identified in case of dipeptides containing C-terminal glycine (X-Gly) (see Fig. 1a.). The metal ion speciation of the palladium(II) complexes of the dipeptides
(Gly-X) (see Fig. 1b.) are similar, but the 4N-species, [Pd(LH-1)2]2- were not formed in this case.
Palladium(II) complexes of triglycine and tetraglycine have also been studied and in contradiction with previous findings the formation of both mono- and bis-complexes was concluded.
The results revealed that in case of palladium(II) even the non-coordinating side-chain residues effect very significantly the metal binding ability of peptides, as it is demonstrated by Figures 1a and 1b, which show the metal ion speciation of the chloride free systems.
 | |
| 1.a. AlaGly/Pd(II) = 2 | 1.b. GlyAla/Pd(II) = 2 |
