S. Kemény, Á. Drégelyi-Kiss, K. Komka, A. Deák
Budapest University of Technology and Economics, Department of Chemical
Engineering,
H-1521 Budapest, e-mail: kemeny.vmt@chem.bme.hu
The within-laboratory fluctuation may cover the existing bias between laboratories. On the other hand, by reducing measurement uncertainty unimportant differences may become visible.
The usual practice specifies tolerable deviation between the two laboratories, most frequently in percentages, independently of the consideration of measurement uncertainty.
In the language of the mathematical statistics the question may be formulated as the need for a proper design, which allows observing relevant differences at high probability, and an acceptance procedure for deciding if the difference between the two laboratories is significantly larger than it is allowed.
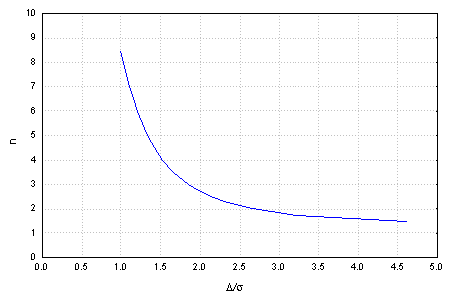
In a simple comparative experiment, where the two-sample t-test is the acceptance procedure, the required number of repetitions for safe detection of a certain bias is calculated through the power function of the test.
The probabilities for errors of first and second kind should be specified more generally, it may be a point of the company policy. The relevant bias to be detected should depend on the experienced uncertainty of the method and the release criteria, and is obviously different for a contaminant and for an active ingredient.
The philosophy is extended to more complex cases such as those involving several batches and/or several analysts within a laboratory.
Generally the number of required repetitions is plotted against the difference to be detected for fixed a and b error probabilities.
